Next Generation Sequencing

Next Generation Sequencing
Next generation sequencing (NGS), massively parallel or deep sequencing are related terms that describe a DNA sequencing technology which has revolutionised genomic research. The principle behind Next Generation Sequencing (NGS) is similar to that of Sanger sequencing, which relies on capillary electrophoresis. The genomic strand is fragmented, and the bases in each fragment are identified by emitted signals when the fragments are ligated against a template strand. While the Sanger method only sequences a single DNA fragment at a time, NGS is massively parallel, sequencing millions of fragments simultaneously per run. Using NGS an entire human genome can be sequenced within a single day. In contrast, the previous Sanger sequencing technology, used to decipher the human genome, required over a decade to deliver the final draft.
There are a number of different NGS platforms using different sequencing technologies. However, all NGS platforms perform sequencing of millions of small fragments of DNA in parallel. Bioinformatics analyses are used to piece together these fragments by mapping the individual reads to the human reference genome. Each of the three billion bases in the human genome is sequenced multiple times, providing high depth to deliver accurate data and an insight into unexpected DNA variation. NGS can be used to sequence entire genomes or constrained to specific areas of interest, including all 22,000 coding genes (a whole exome) or small numbers of individual genes.
Advantages of NGS include: Higher sensitivity to detect low-frequency variants. Faster turnaround time for high sample volumes. Comprehensive genomic coverage.
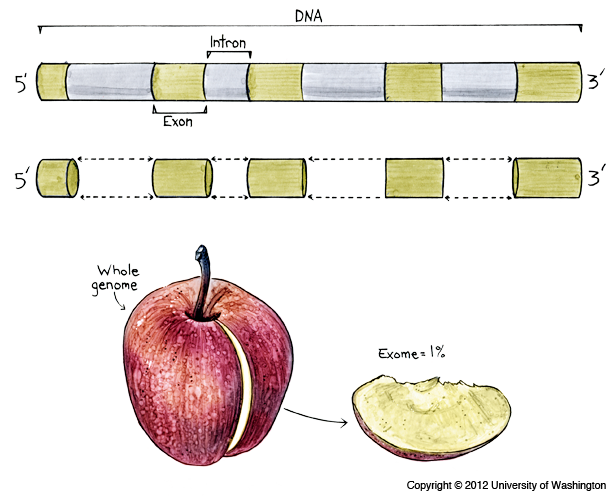
Whole Exome / Genome sequencing
Determining the order of DNA building blocks (nucleotides) in an individual's genetic code, called DNA sequencing, has advanced the study of genetics and is one technique used to test for genetic disorders. Two methods, whole exome sequencing and whole genome sequencing, are increasingly used in healthcare and research to identify genetic variations; both methods rely on new technologies that allow rapid sequencing of large amounts of DNA. These approaches are known as next-generation sequencing (or next-gen sequencing).
With next-generation sequencing, it is now feasible to sequence large amounts of DNA, for instance all the pieces of an individual's DNA that provide instructions for making proteins. These pieces, called exons, are thought to make up 1 percent of a person's genome. Together, all the exons in a genome are known as the exome, and the method of sequencing them is known as whole exome sequencing. This method allows variations in the protein-coding region of any gene to be identified, rather than in only a select few genes. Because most known mutations that cause disease occur in exons, whole exome sequencing is thought to be an efficient method to identify possible disease-causing mutations.
However, researchers have found that DNA variations outside the exons can affect gene activity and protein production and lead to genetic disorders--variations that whole exome sequencing would miss. Another method, called whole genome sequencing, determines the order of all the nucleotides in an individual's DNA and can determine variations in any part of the genome.
While many more genetic changes can be identified with whole exome and whole genome sequencing than with select gene sequencing, the significance of much of this information is unknown. Because not all genetic changes affect health, it is difficult to know whether identified variants are involved in the condition of interest. Sometimes, an identified variant is associated with a different genetic disorder that has not yet been diagnosed (these are called incidental or secondary findings).
In addition to being used in the clinic, whole exome and whole genome sequencing are valuable methods for researchers. Continued study of exome and genome sequences can help determine whether new genetic variations are associated with health conditions, which will aid disease diagnosis in the future.
